
Key Resources

Stead Family Department of Pediatrics' Research Day will be held from 12 noon to 5:30 p.m. CST on Friday, April 7, 2023!
Please plan to attend as you are able to support and learn about the impressive research activities in the department. All activities except the poster session will be held in 2JCP Pediatrics Conference Center, with transition to the MERF atrium for posters. The festivities will start with box lunches available in 2JCP Conference Center area and in the MERF auditorium.
At 12:00 p.m. CST, Dr. Raphaela T. Goldbach-Mansky from the National Institute of Allergy and Infectious Disease (NIAID) will join us in person and virtually (streamed in the Conference Center), presenting a talk entitled “The power of de novo gain-of function mutations in revealing innate immune dysfunction ...one child at a time.”
Three of our pediatric faculty members will present their research from 1:15 to 2:15 p.m., followed by the work of three outstanding senior pediatrics fellows. The Data Blitz from 3:00-3:45 p.m. will include selected projects by trainees of all levels, with each project presented in one minute.
Please join us for the poster session from 4:00 to 5:30 p.m. CST in the MERF atrium, where snacks will be available. We have approximately 50 posters for viewing! Posters will be judged by faculty, and prizes awarded at the end of the session. Come enjoy seeing your friends and colleagues and congratulate the presenters on their hard work, and learn about the exciting work being done in the department.

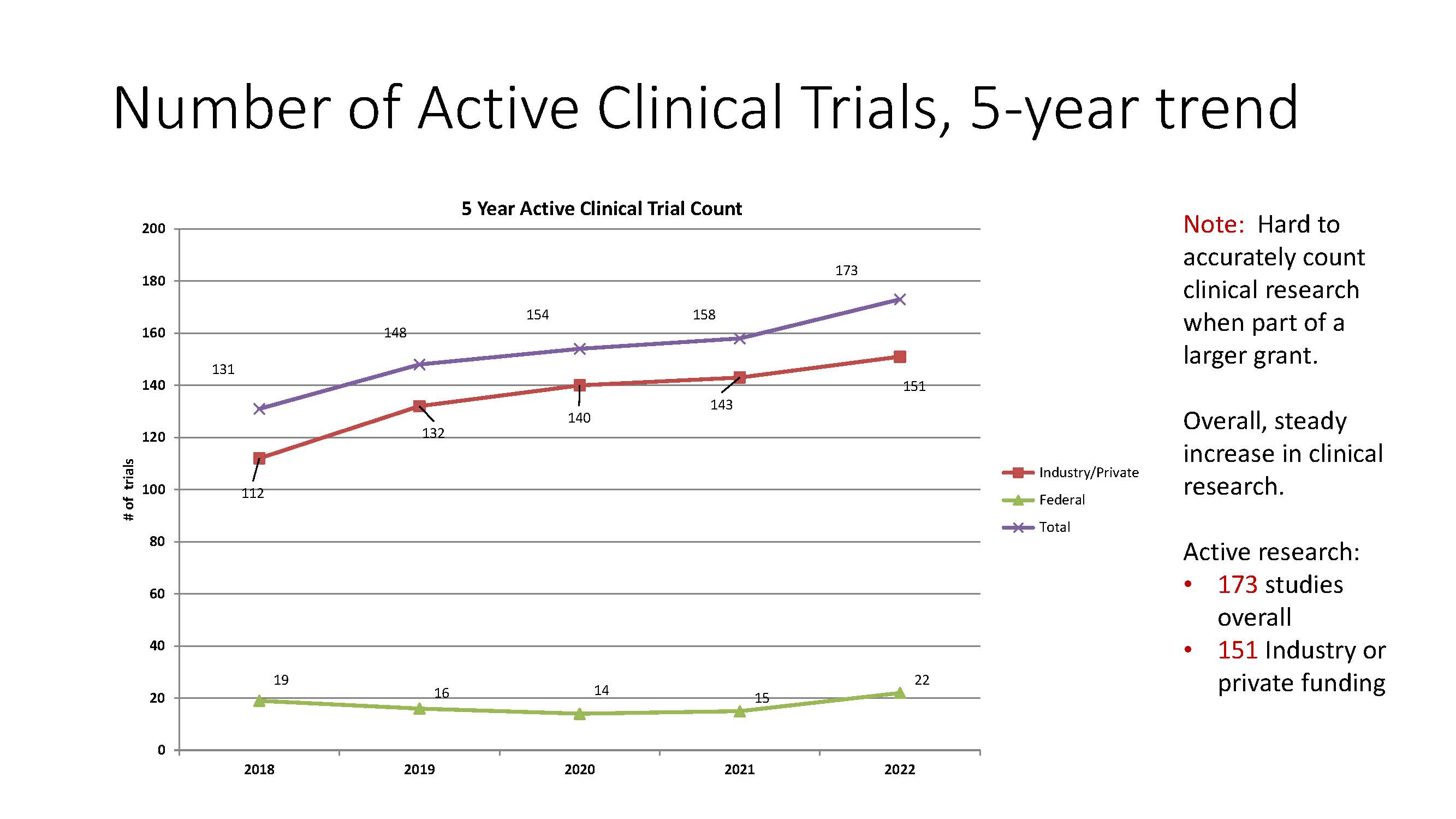
In this newsletter, we present data about the Stead Family Department of Pediatrics' participation in clinical research, focused on the past five years, through fiscal year 2022.
Clinical research can be defined as research that involves human participants; thus, it encompasses a wide range of activities. Clinical research includes natural history studies that lay the groundwork for new therapies, through interventional clinical trials. Clinical research brings patients to the University from across the country and makes us stand apart from our regional healthcare peers. Through research participation, we can offer our patients the opportunity to advance knowledge, to improve clinical care, and to receive cutting edge potential therapies.
Funding for clinical research can come from federal sources (NIH, FDA, CDC, DOD, etc.), foundations/private funds, or from industry. Most later stage therapeutic trials are directed by industry due to their cost and complexity.
Clinical coordinators are critical to all aspects of clinical trial operations, and the department has ~40 clinical trial coordinators supporting the studies.
Caveats:
- In some the information presented here, we have attempted to identify non-industry sponsored studies that qualify as clinical research but might have missed some or included some that don’t really involve human research. When grants include both bench and human research components it is particularly difficult to classify them. We have therefore included these in counts of studies, but not in the financial data.
- These data include only grants that are primarily managed in the Department of Pediatrics, thus multiple PI, sub-PIs, and collaborations are not included if the grant is managed through a different department, even if financial support is provided to a pediatric PI.
Key points:
- Steadily increasing number of clinical trials: 173 studies overall and 151 Industry or private funding trials in FY 2022.
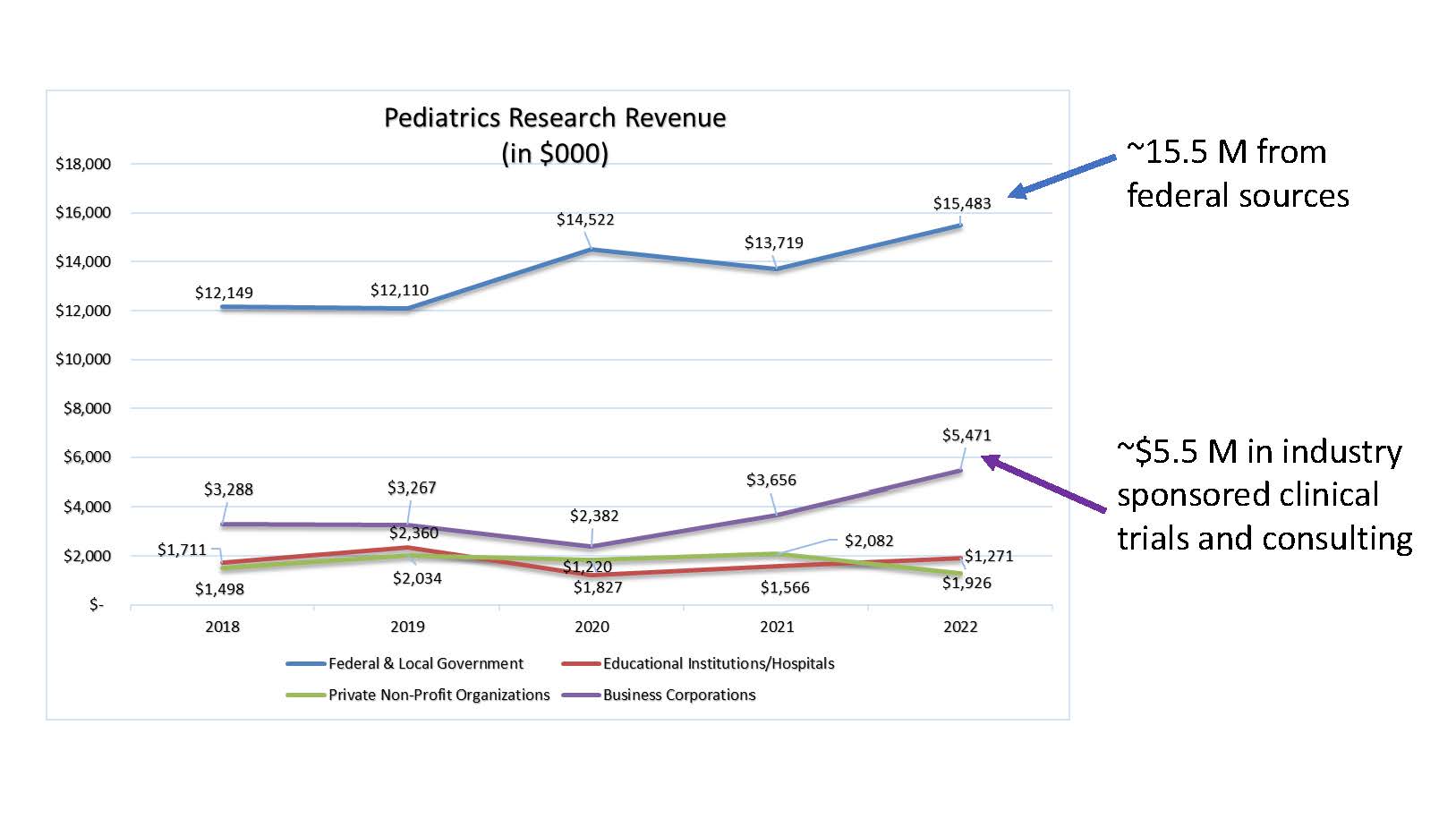
- Increasing revenue received from both federal and industry-sponsored clinical research.
- Clinical trial revenues require a balance of considerations to best serve our patients.
- Making excess money that comes out of our patients’ pockets indirectly.
- Having the funds to maintain a robust research program, that ultimately improves outcomes for patients.


Here are a few things to keep in mind when requesting Epic data:
Submit requests in ESC as far as possible in advance of any deadline as possible.
We use ESC as our official work queue for data requests and we review and prioritize everything in the ESC queue weekly. A long lead time allows time for analysis, development, and testing. It’s also helpful if you note in the “brief summary” section if the deadline is a “hard” deadline (e.g., data needs to be submitted by a specific date to a publication) or a “soft” deadline (e.g., you would like to have the data by a certain date, but that date is flexible).
Be sure to mention “Pediatrics” somewhere in the “brief summary” section in the ESC request.
This will ensure it gets assigned to the right resource. (This is also noted in the ESC steps.)
We’ll use the ESC ticket for communication and documentation.
Important emails, meeting summaries, requirements documents (screenshots, for example) will all be stored in the ESC ticket. It’ll all be accessible to you so that you can easily see the status and past activity. And when the request is completed, the code used to generate the data will be attached. That way, if there is a need to rerun the data the exact code that was used to create the original dataset will be available.
Don’t worry about trying to describe a solution
In other words, you don’t need to try to figure out if you want it in Slicer/Dicer, Reporting Workbench, Tableau, Excel, or some other tool. The first thing we’ll do once we start work on your request is have in-depth discussion with you about the data you need. Once we understand the details, we’ll do the necessary work to determine the best tool for your request.
